A desperately needed tool to curb the COVID-19 pandemic is an inexpensive home-based rapid testing kit that can detect the coronavirus without needing to go to the hospital.
The Food and Drug Administration has approved a few home sample collection kits but a number of researchers, including myself, are using the gene-editing technique known as CRISPR to make home tests. If they work, these tests could be very accurate and give people an answer in about an hour.
I am a biomolecular scientist with training in pharmaceutical sciences and biomedical engineering and my lab focuses on developing next-generation of technologies for detecting and treating cancer, genetic and infectious diseases.
The COVID-19 disease is caused by a coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Unlike humans which carry their genetic material encoded in DNA, the coronavirus encodes theirs in a related molecule called RNA.
My research group recently engineered a sensitive CRISPR-based technology, that we named CRISPR-ENHANCE, and used it to create a rapid test for SARS-CoV-2 RNA. Our assay works like a pregnancy test and shows two purple colored lines if the sample is positive for the virus. Using our technology, I envision developing a test kit that would allow rapid detection of SARS-CoV-2 RNA in saliva within 45-60 minutes at home without needing any expensive equipment.
Current landscape of coronavirus testing
The FDA recently gave a green light to a couple of sample collection kits from LabCorp and Everywell under the Emergency Use Authorization (EUA) that would allow people to ship out the nasal swab samples for analysis. Patients can take a swab of their nose, ship the samples to a lab, and wait for a few days to get the results back.
Although not an at-home testing kit, the test allows the samples to be shipped directly to a lab for detecting SARS-CoV-2 RNA. There they use a technique called reverse transcription-polymerase chain reaction (RT-PCR), which converts the viral RNA into DNA so that it can be easily multiplied and detected.
Although most FDA-approved tests are based on detecting SARS-CoV-2 RNA at an early stage, before symptoms even appear, such tests can only be performed in a laboratory setting with expensive equipment and can take multiple days to get the results.
Several antibody testing kits have been approved by the FDA that use a paper-based lateral flow strip, also similar to an at-home pregnancy testing strip, for detecting antibodies called IgM and IgG. Almost all SARS-CoV-2 infected patients make antibodies within 19 days of onset of symptoms and then the body continues to make detectable antibodies for several weeks to months even after symptoms fades away. Therefore, the Centers for Disease Control and Prevention recommends using antibody tests for detecting past infections.
However, the coronavirus is usually very active and contagious in the first week of infection and peaks on the day of onset of symptoms. Therefore, to prevent the spread of coronavirus, it is extremely important to detect coronavirus early to block the spread.
The antibody testing can be great for detecting past infections but they cannot reliably detect current or early infections. The delayed appearance and patient-to-patient variability of antibodies in a blood test further complicates the COVID-19 diagnosis with antibody testing kits.
In addition, the variability between different antibody testing methods have raised doubts about the reliability of these test kits.
Therefore, the National Institutes of Health recently announced a Rapid Acceleration of Diagnostics (RADx) which offers up to US$500 million in funding for ramping up the technologies that detect the SARS-CoV-2 virus.
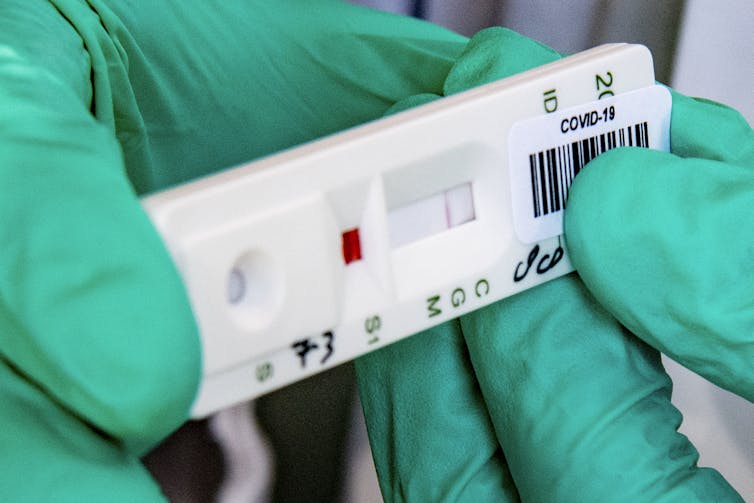
How CRISPR-based diagnostics work
Most people know of CRISPR/Cas systems as a famous gene-editing technology that can precisely edit DNA. Researchers engineer a “guide” RNA molecule with a target genetic sequence that serves like a GPS and zooms in on a location on the DNA where a Cas protein, a pair of molecular scissors, can cut at the desired location.
Scientists in the labs of Feng Zhang at MIT, Jennifer Doudna at UC Berkeley and others discovered several newer versions of CRISPR/Cas systems, including ones using the proteins Cas12a and Cas13a-d, which get crazy cutting once they find their match.
My colleagues and I have used this Cas12a-based CRISPR technique to detect the coronavirus.
The coronavirus RNA activates CRISPR/Cas, transforming a pair of controlled molecular scissors into an unstoppable chainsaw. When the the CRISPR/Cas enzyme activates, we know that the genetic sequence of the coronavirus is present in the saliva sample. To make the signal of the coronavirus stronger in the testing kit, we add millions of synthetic “reporter molecules” which are also chopped up by the CRISPR/Cas mechanism. This means that within minutes we can detect detect the presence of coronavirus.
Under EAU, the FDA recently approved the first CRISPR-based SARS-CoV-2 RNA testing kit from Sherlock Biosciences for testing nasal swabs in a lab. Although not yet approved for at-home testing, this is a big leap toward the development of CRISPR-based diagnostics.
While similar CRISPR-based test kits are in development including one from Mammoth Biosciences and others, our CRISPR-ENHANCE technology relies on engineered CRISPR RNAs that increases the speed of Cas12a “chainsaw” by between three- and four-fold.
This technique dramatically enhances the sensitivity of detection. Our system can detect fewer virus in a clinical sample faster with a clear visual readout. We are in the process of clinically validating the CRISPR-ENHANCE technology for SARS-CoV-2 RNA detection.
Increasing the speed of CRISPR diagnostics
Standard collection method for detecting respiratory viruses in the clinic is the nasal swab. However, coronaviruses have been detected at comparable levels in saliva so some researchers are now turning to saliva for diagnostic testing.
Collecting saliva is not only less invasive than the nasal swabs but also contains more virus, which makes it easier to detect with RT-PCR. In fact, an at-home saliva collection kit just received a green light by the FDA on May 8, 2020. In our validation study we will be internally comparing our test between the nasal swabs and saliva for FDA approval.
We are developing a six-step procedure for home-based testing for saliva along with the nasal swabs. Here is how it would work with saliva.
Spit into a sample collection tube that contains dry chemical reagents that will begin to react with your saliva when you drop the closed tube into the warm water for 30 minutes.
The heat helps the chemicals break up the virus particle and expose the virus’s genetic material – RNA. The RT-PCR reagents basically multiply the viral RNA creating billions of copies, which are more easily detected.
After 30 minutes, transfer the contents of the collection tube to a second tube containing dried CRISPR components and leave it at room temperature for 10-15 minutes.
Only if CRISPR/Cas finds the specific coronavirus RNA, will it become active and chop up the synthetic reporter molecules that are engineered and added to this second tube. This part happens in just six minutes.
We then drop a paper strip into the second tube. Within 30 seconds one or two purple bands reveal the results.
The health care provider can then direct the individual to either quarantine, isolate and/or recommend further testing such as antibody-based tests. In our study, currently under peer review, we demonstrated that the ENHANCE technology itself is versatile and can also be adopted for detecting a range of targets including HIV, HCV and prostate cancer.
While there are several labs and companies are rushing to develop similar CRISPR-based coronavirus detection kits for saliva testing, we believe our approach offers the fastest detection. We hope to bring the cost of the kit down to between $1 and $2 so that developing countries can also afford a rapid and reliable coronavirus testing kit.
[You need to understand the coronavirus pandemic, and we can help. Read The Conversation’s newsletter.]
Post a Comment